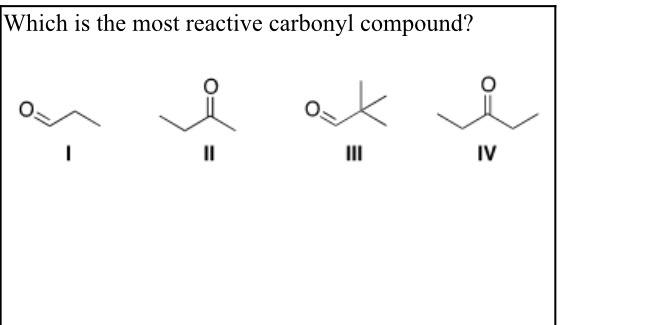
Which Is The Most Reactive Carbonyl Compound . The enolic form acetone contains : A) i b) ii c) iii d) iv.
Solved Which Is The Most Reactive Carbonyl Compound? Iii Iv | Chegg.com from www.chegg.com
A) benzophenone b) acetophenone c) benzaldehyde d) ethanal ans (iv). Rr' o o nu + nu rr' o o nu or + nu addition of heteroatom nucleophiles to carbonyl compounds:
Solved Which Is The Most Reactive Carbonyl Compound? Iii Iv | Chegg.com
Which of the following is the most reactive carboxylic acid derivative? If the carbonyl compound is a ketone, we use the suffix. There might be many concepts out there, that explain this order, the above linked question could only halfway.
Source: polymerdatabase.com
Since, among all the groups attached has maximum electron withdrawing effect, electron deficiency will be maximum at carbonyl carbon of acetyl chloride. In aldehydes, the carbonyl group is bonded to a carbon and hydrogen while in ketones, it is bonded to two carbon atoms. A) benzophenone b) acetophenone c) benzaldehyde d) ethanal ans (iv).
Source: www.doubtnut.com
If the carbonyl compound is a ketone, we use the suffix. It does not reduce tollen’s reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. Is most reactive to nucleophilic attack at carbonyl group
Source: www.chegg.com
Is most reactive to nucleophilic attack at carbonyl group Which is the most reactive carbonyl compound? This functional group can participate in multiple modes of reactions.
Source: www.coursehero.com
Rank the following compounds in order of increasing reactivity (least to most reactive) with respect to acyl substitution. It does not reduce tollen’s reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. Because the grignard reagent will react with the acid and be quenched b.
Source: chemistry.stackexchange.com
It is the enol, not the carbonyl compound, which is reactive toward bromine. The carbonyl carbon in the ketone is a little more stable than the carbonyl carbon in the aldehyde. Acyl halide > acid anhydride > aldehyde > ketone > ester ~ carboxylic acid > amide > carboxylate ion.
Source: chem.libretexts.org
This is because the enol is so reactive toward bromine that it never has a chance to reverse step 2, i.e., it is not protonated by hydronium ion to give back the conjugate acid of the. This is kind of like asking “which is faster, a rocket or a computer?” the reactions of alkenes and ketones are fundamentally different which.
Source: www.youtube.com
The molecular mass of the compound is 86. Which is the most reactive carbonyl compound? However, the term ‘carbonyl’ can also refer to carbon monoxide as the ligand within an organometallic or inorganic compound (say a metal carbonyl, such as nickel carbonyl).
Source: www.chegg.com
Acyl halide > acid anhydride > aldehyde > ketone > ester ~ carboxylic acid > amide > carboxylate ion. Therefore from the above explanation we can say that ethanol will be most reactive towards nucleophilic addition reaction. This is because the enol is so reactive toward bromine that it never has a chance to reverse step 2, i.e., it is.
Source: www.toppr.com
Reactions of carbonyl compounds the most important mechanistic feature of all reaction involving c=o (aldehydes, ketones, derivatives of carboxylic acids) is the addition of a nucleophile = formation of the tetracoordinate c intermediate: Which among the following isomeric compounds is most reactive? Which is the most reactive carbonyl compound?
Source: www.chegg.com
Therefore from the above explanation we can say that ethanol will be most reactive towards nucleophilic addition reaction. ¥the sp2 hybridization of the carbonyl compound means that attack of the nucleophile on the carbonyl carbon may occur from either face. The carbonyl carbon in the ketone is a little more stable than the carbonyl carbon in the aldehyde.
Source: www.masterorganicchemistry.com
This is kind of like asking “which is faster, a rocket or a computer?” the reactions of alkenes and ketones are fundamentally different which makes the comparison meaningless. If the carbonyl compound is a ketone, we use the suffix. Because the ketone will be protonated and thus unreactive c.
Source: www.chegg.com
Therefore from the above explanation we can say that ethanol will be most reactive towards nucleophilic addition reaction. Which is the most reactive carbonyl compound? Because the ketone will form an unreactive enol d.
Source: www.chegg.com
The carbonyl compounds in which carbonyl group is bonded to oxygen are known as carboxylic acids, and their derivatives (e.g. Hence the correct answer is (b). If the carbonyl compound is a ketone, we use the suffix.
Source: www.coursehero.com
This is because the enol is so reactive toward bromine that it never has a chance to reverse step 2, i.e., it is not protonated by hydronium ion to give back the conjugate acid of the. There might be many concepts out there, that explain this order, the above linked question could only halfway. A) benzophenone b) acetophenone c) benzaldehyde.
Source: www.chegg.com
Hence the correct answer is (b). A) benzophenone b) acetophenone c) benzaldehyde d) ethanal ans (iv). Reactions of carbonyl compounds the most important mechanistic feature of all reaction involving c=o (aldehydes, ketones, derivatives of carboxylic acids) is the addition of a nucleophile = formation of the tetracoordinate c intermediate:
Source: www.chegg.com
In aldehydes, the carbonyl group is bonded to a carbon and hydrogen while in ketones, it is bonded to two carbon atoms. The carbonyl carbon in the ketone is a little more stable than the carbonyl carbon in the aldehyde. On vigorous oxidation it gives ethanoic and propanoic acid.
Source: www.chegg.com
The carbonyl compounds in which carbonyl group is bonded to oxygen are known as carboxylic acids, and their derivatives (e.g. One of two suffixes in common names may indicate the presence of a carbonyl group in a molecule. Here most reactive functional group is we have to choose.
Source: www.toppr.com
There are two important factors that help in comparing the reactivity of aldehydes and ketones in nucleophilic addition reaction and these are steric factor and the electronic factor. Here out of four compound 2 are aldehyde (i & iii) a. Which is the most reactive carbonyl compound?
Source: www.chegg.com
The major product in the reaction: A) i b) ii c) iii d) iv. Therefore from the above explanation we can say that ethanol will be most reactive towards nucleophilic addition reaction.
Source: www2.chemistry.msu.edu
The carbonyl compounds in which carbonyl group is bonded to oxygen are known as carboxylic acids, and their derivatives (e.g. Which is the least reactive carbonyl compound? This is because the enol is so reactive toward bromine that it never has a chance to reverse step 2, i.e., it is not protonated by hydronium ion to give back the conjugate.