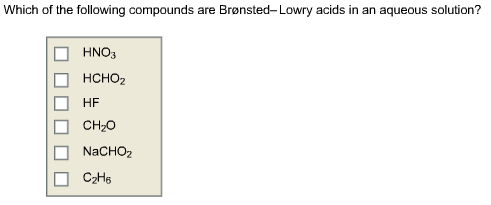
Which Of The Following Is A Bronsted Lowry Acid. First, let us define the b.l. It will not make many compounds.
 Solved Which Of The Following Cannot Act As A Bronsted-Lowry | Chegg.com from www.chegg.com
Solved Which Of The Following Cannot Act As A Bronsted-Lowry | Chegg.com from www.chegg.com
H 2 s ⇌ h s − + h +. A bronsted lowry acid is defined simply as a proton donor.
Solved Which Of The Following Cannot Act As A Bronsted-Lowry | Chegg.com
Hno 2 b) h 2 so 3; Its conjugate acid is the ammonium ion. So it is a bronston lowry base.