Which Of The Following Is The Empirical Formula For C4H8 . A)c2n2h8 b)sb4s6 c)be2(cr2o7)2 d) c3h8o Aniline, consists of c, h, and n.
Structural Formula (C4H8) Having The Same Molecular Formula | Download Scientific Diagram from www.researchgate.net
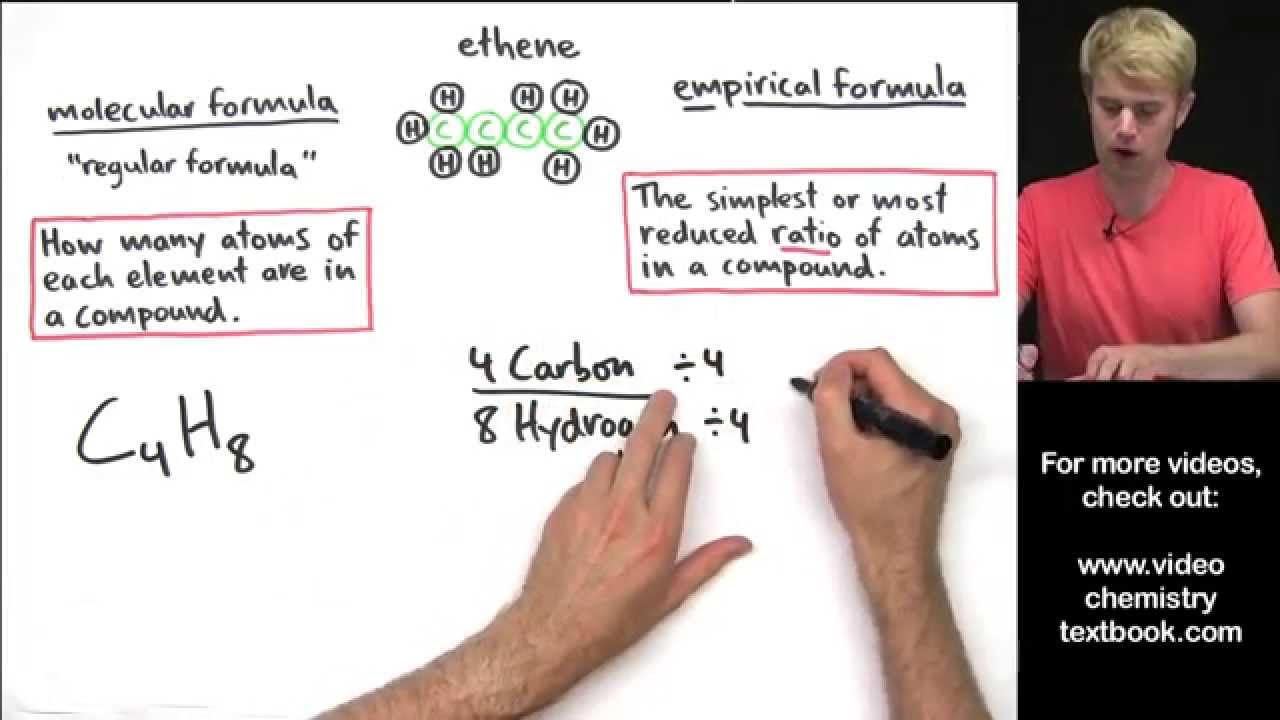
4:8 ≈ 1:2 c 4 h 8 ÷ 4 = ch 2 empirical formula mass: Which of the following shows an empirical formula?
Structural Formula (C4H8) Having The Same Molecular Formula | Download Scientific Diagram
4:8 ≈ 1:2 c 4 h 8 ÷ 4 = ch 2 empirical formula mass: Is c4h8 an empirical formula? There is no number with which we can divide the ratio further to get a simpler formula hence the molecular formula of the given compound is the empirical formula of the compound.
Source: slideplayer.com
C = 12.01 g/mol h =. Here (en)n = m f = (ch 2o) × 3 = c3h 6o3 as required. What is the molecular formula of each compound?
Source: studylib.net
A)c2n2h8 b)sb4s6 c)be2(cr2o7)2 d) c3h8o Ethylene is c2h4, or twice the empirical formula….empirical formulach (92.2% c; The number of structural isomers possible for c4h8 is 1 4 2 3 3 5 4 6.
Source: www.quora.com
Butene is c4h8, or four times the empirical formula; The molecular formula is always a mulitple of the empirical formula. Molar mass of c4h8 = 56.10632 g/mol.
Source: www.toppr.com
The molecular formula is always a mulitple of the empirical formula. 12.0107*4 + 1.00794*8 ›› percent composition by element If the combustion of 9.71 mg of aniline yields 6.63 mg h2o and 1.46 mb n2, what is the empirical formula?
Source: www.slideserve.com
There are a number of types of isomers. Here (en)n = m f = (ch 2o) × 3 = c3h 6o3 as required. 18) zinc (65.4 g/mol) and sulfur (32.1 g/mol) react to form.
Source: www.quora.com
What is the empirical formula and empirical formula mass for each of the following compounds. (remember, only look at the number of carbons) 3. There is no number with which we can divide the ratio further to get a simpler formula hence the molecular formula of the given compound is the empirical formula of the compound.
Source: www.chegg.com
What is the molecular formula of a compound with the molecular weight of 372.504 amu? Combustion of such compounds yields co2, h2o, and n2. • the molecular formula is the true or actual ratio of the atoms in a compound.
Source: quizlet.com
4:8 ≈ 1:2 c 4 h 8 ÷ 4 = ch 2 empirical formula mass: Molar mass of c4h8 = 56.10632 g/mol. There is no number with which we can divide the ratio further to get a simpler formula hence the molecular formula of the given compound is the empirical formula of the compound.
Source: slideplayer.com
What is the empirical formula for c8h14? Butenes are unsaturated olefinic hydrocarbons, c4h8, mw 56.1080, existing in four isomers: What is the empirical formula and the empirical formula mass for each of the following compounds:
Source: www.elevise.co.uk
May 4, 2021 by admin. There are a number of types of isomers. 17) each of the following is a valid molecular formula.
Source: www.youtube.com
• the molecular formula is the true or actual ratio of the atoms in a compound. Is c4h8 an empirical formula? (2) (total 5 marks) chlorine can be used to make chlorinated alkanes such as dichloromethane.
Source: slideplayer.com
Combustion of such compounds yields co2, h2o, and n2. (a) c2h4 (b) c2h6o2 (c) n2o5 (d) ba3(po4)2 (e) te4l16. Timberlake lectureplus 3 • an empirical formula represents the simplest whole number ratio of the atoms in a compound.
Source: www.slideserve.com
17) each of the following is a valid molecular formula. Which of the following shows an empirical formula? 16) which of the following compounds does not have the same empirical formula as the others?
Source: www.researchgate.net
A)c2n2h8 b)sb4s6 c)be2(cr2o7)2 d) c3h8o If the combustion of 9.71 mg of aniline yields 6.63 mg h2o and 1.46 mb n2, what is the empirical formula? What is the empirical formula for c8h14?
Source: www.quora.com
Prefix (# of carbons) + ending (type of h.c.) the endings used for the different types of hydrocarbons are: If the combustion of 9.71 mg of aniline yields 6.63 mg h2o and 1.46 mb n2, what is the empirical formula? Which of the following is the empirical formula for c4h8?
Source: www.slideserve.com
Chemistry q&a library write the empirical formula for the following compounds c4h8 h2o2 co2 There are a number of types of isomers. Prefix (# of carbons) + ending (type of h.c.) the endings used for the different types of hydrocarbons are:
Source: www.chegg.com
Most empirical formulas for compounds are identitical to its molecular formula different compounds can have the same empirical formula examples : What is the empirical formula and empirical formula mass for each of the following compounds. If the combustion of 9.71 mg of aniline yields 6.63 mg h2o and 1.46 mb n2, what is the empirical formula?
Source: www.chegg.com
Which of the following shows an empirical formula? You’re just simplifying c4h8, 4 can go into c4 1 time (so we just say c) and 4 can go into h8 2 times (h2) A)c2n2h8 b)sb4s6 c)be2(cr2o7)2 d) c3h8o
Source: www.researchgate.net
First off, would this be written like: The empirical formula of pvc is the same as the empirical formula of its monomer. • the molecular formula is the true or actual ratio of the atoms in a compound.
Source: socratic.org
What is the empirical formula for c8h14? Mass of co2 given in the problem is 8.5900g , convert the mass to moles by dividing mass in grams with the molar mass of co2. The empirical formula of pvc is the same as the empirical formula of its monomer.